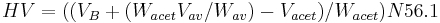Hydroxyl value
In chemistry, the hydroxyl value is a measure of the content of free hydroxyl groups in a compound, typically a fat, oil, or natural or synthetic ester. This value is a useful measure of the degree of esterification in ester synthesis. The method involves acetylation of the free hydroxyl groups of the compound with acetic anhydride in pyridine solvent [1]. After completion of the reaction, water is added, the remaining acetic anhydride is converted to acetic acid, and measured by titration with potassium hydroxide. Other functional groups, such as primary or secondary amines, will be reported as hydroxyl. It is expressed as the mass of potassium hydroxide (KOH) in milligrams equivalent to the hydroxyl content of one gram of the chemical substance, corrected for carboxyl hydroxyl groups by titration of an unacetylated sample of the same material.
Where VB is the amount of titrant (ml) consumed by the blank, at the equivalent point, Wacet is the weight of sample (in grams) used for acetylation, Vav is the amount of titrant used for determining the acid value, Wav is the weight of sample used for determining the acid value, Vacet is the amount of titrant used for the acetylated sample, N is the normality of the titrant, and 56.1 is the molecular weight of KOH.
The standard method for determining the hydroxyl value is ASTM D 1957.